Research
Energy-Coupling Factor (ECF) transporters
ECF transporters are members of the ATP-binding cassette (ABC) transporter superfamily. The term "energy-coupling factor" was coined in the 1970s by american researchers while analyzing vitamin uptake into lactic acid bacteria. These investigators uncovered vitamin-binding integral membrane proteins as part of the transporters and realized that those binding proteins depend on an unknown component, named energy-coupling factor, for transport activity. Our research in collaboration with scientists from Russia, the United States and the Netherlands identified the ECF as an assembly of two ABC ATPases (or nucleotide-binding domains) and a coupling integral membrane protein (T component). These findings stimulated a large body of research within the last years resulting in structural and functional characterization of several ECF transporters. Those transporters are essential for micronutrient uptake in a variety of bacterial pathogens with restricted biosynthetic capacity.
Mechanistic types of ECF transporters
ECF transporters operate by a rotation mechanism of the S components. ATP binding leads to release of the S component among subclass II systems (in which a single ECF operates together with different S components for individual vitamins; Fig. 1B) and perhaps also among metal-specific ECF transporters (Fig. 1C). A comparable release-and-catch mechanism has not been observed in our analyses of an ECF-type biotin transporter with dedicated components (Fig. 1A).

Fig. 1. Simplified mechanistic models for ECF transporters. ATP induces rotation of the S component. (A) The subclass I biotin transporter BioM2NY does not dissociate during the transport cycle. (B) Release of the S component among subclass II ECF transporters. (C) Additional components are required by metal-specific ECF transporters. Mechanistic details are enigmatic.
[Published in Finkenwirth, F. and T. Eitinger. 2019. ECF-type ABC transporters for uptake of vitamins and transition metal ions into prokaryotic cells. Res. Microbiol. 170:358-365.]
Features of the substrate-binding integral membrane proteins (S components)
S components for different substrates share a high degree of structural similarity in the absence of amino acid sequence identity (Fig. 2).
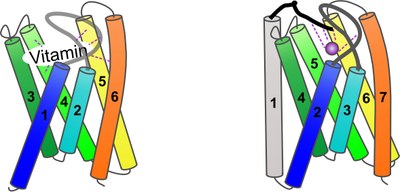
Fig. 2. Cartoon representation of S components for vitamins (left panel) and transition metal cations (right panel). The overall structure of S components is similar. Vitamins are bound by ligands from transmembrane helices 4, 5 and 6, and in most cases by extracytoplasmic loops. Metal-specific S components contain an additional N-terminal transmembrane helix and a strongly conserved N-terminus that provides three metal ligands.
[Published in Finkenwirth, F. and T. Eitinger. 2019. ECF-type ABC transporters for uptake of vitamins and transition metal ions into prokaryotic cells. Res. Microbiol. 170:358-365.]
CbiMQO2-CbiN – a cobalt transporter as model system for metal-specific ECF transporters
We are aiming at a better understanding of the essential role of the helper protein CbiN in activation of the CbiMQO2 core transporter. Distinct but fleeting loop-loop interactions as indicated in Fig. 3 are a major clue. Those interactions have been identified by site-pecific crosslinking upon cysteine-scanning mutagenesis. They reduce mobility of the CbiN loop as evidenced by electron paramagnetic resonance (EPR) spectroscopy upon site-specific spin labeling on one hand. On the other hand, they contribute to dynamics of the CbiM-CbiN interaction as observed by magic-angle-spinning solid-state nuclear magnetic resonance (MAS ssNMR) spectroscopy of isotope-labeled protein reconstituted in proteoliposomes.
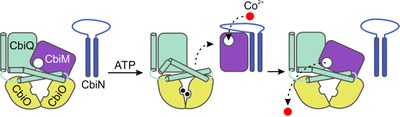
Fig. 3. Hypothetical transport mechanism of the CbiMQO2 holotransporter. Binding of ATP to the nucleotide- and metal-free holotransporter leads to closure of the ATPase dimer and uplifting of the S component CbiM. Release of CbiM as has been observed for S components of subgroup II ECF transporters is proposed, since our data suggest mutually exclusive interaction of CbiM with either CbiQ or CbiN. Helix-helix and in particular loop-loop interactions between CbiM and CbiN facilitate in an unknown manner selective metal loading to CbiM. Alternate scenarios are conceivable for the final steps. Metal-loaded CbiM may recombine with the CbiQO2 energy-coupling factor prior to or after ATP hydrolysis and topple over. Displacement of extracytoplasmic loop 2 destroys the metal-binding site and leads to substrate release.
[Published in Finkenwirth, F., M. Sippach, S.N. Pecina, M. Gäde, J. Ruta, A. Ricke, E. Bondarenko, J.P. Klare, M. Zinke, S. Lange, A. Lange, H.-J. Steinhoff and T. Eitinger. 2020. Dynamic interactions of CbiN and CbiM trigger activity of a cobalt energy-coupling-factor transporter. Biochim. Biophys. Acta – Biomembranes 1862:183114, open access]