GluTR proteolysis by the caseinolytic proteases (Clp) system
Janina Apitz, Boris Hedtke
GluTR is target of the plastid caseinolytic protease (Clp) system (Nishimura et al., 2013, 2015). The Clp selectors ClpS1 and ClpF and the chaperones ClpC1 and ClpC2 bind to the N-terminus of GluTR (Nishimura et al., 2015, Apitz et al., 2016). Mutant analyses indicate that GBP competes with these components of the Clp complex for binding at the N-terminus of GluTR. The hema1 mutant was rescued by expressing GluTR1ΔHBD under the HEMA1 promoter resulting in a wild-type phenotype with elevated content of the aminoterminally truncated GluTR protein.


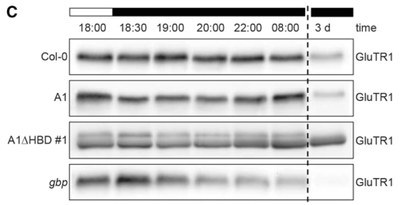
Deletion of the N-terminal heme-binding domain (HBD) of Arabidopsis GluTR1 (scheme in A) results in a wild-type phenotype as shown for two transgenic lines (A1∆HBD in B). Stability of truncated GluTR1 (C, A1∆HBD) is increased when compared to the full-length protein. In contrast, a loss of GBP leads to destabilization of GluTR1 (C). (Figure taken from Apitz et al., Plant Physiol. 2016 Feb 16. pii: pp.01945).
It is suggested that binding of GBP protects GluTR1 from degradation, while the Clp system is responsible for GluTR proteolysis. This control is hypothesized to balance GluTR1 activity during dark periods and, likely, also throughout light exposure.

A model for the role of GBP and the Clp protease activity in regulating GluTR in light- and dark-grown plants (Apitz et al., 2016, Plant Physiol. 2016 Feb 16. pii: pp.01945.2015).
During light exposure (left panel) the main ALA synthesis is suggested to be located in the stroma, while a minor portion of GluTR is attached to the membrane through interaction with GBP. In darkness (right panel), the predominant ALA synthesis is repressed by interaction of GluTR1 with the membrane-localized FLU through an unknown mechanism. The binding of GluTR1/2 to GBP is proposed to enable ongoing ALA synthesis during darkness for continuous heme formation (Czarnecki et al., 2011). Unwanted GluTR1 and GluTR2 in the stroma are degraded through the Clp protease to prevent the accumulation of Pchlide and necrosis upon illumination. The N-terminal HBD of GluTR is indicated in black.