Interfacial Cell Biology
Dr. Roland L. Knorr
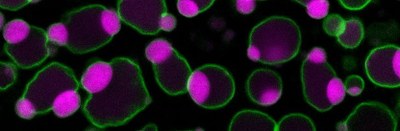
in developing plant embryos. Live-cell imaging of an Arabidopsis thaliana cotyledon. Image size: about 50x100 µm.
The 'Interfacial Cell biology' research group investigates how organelles develop in cells. Particularly, we focus on morphogenic processes that are mediated by contacts between liquid compartments (also known as membrane-less condensates or droplets) and membrane-bound organelles. Our aim is to achieve a comprehensive physico-molecular knowledge of the droplet-membrane interaction, which will inform the precise manipulation of such cellular processes in basic research and applications. The Interfacial cell biology group will focus on these key areas:
Autophagy: The autophagosome is the key organellar component of autophagy, the highly-conserved intracellular bulk degradation pathway. During autophagy, autophagosomes isolate cellular material within curving membrane sheets, which are then delivered to the lysosome/vacuole for degradation and metabolite recycling. Recently, we discovered that phase separated droplets mediate autophagosome formation via wetting. This complex mechanism is controlled by droplet and membrane properties and results in several distinct pathways of recycling material selection and autophagosomal morphologies (Nature 2020, Nature 2021, Autophagy 2021).
Embryogenesis: Most protein eaten by humans and animals originates from plants, in particular seeds including soy bean and wheat. During their development, seeds accumulate protein in dedicated organelles that are known as protein storage vacuoles. Recently, we discovered that micrometer-sized liquid droplets containing storage proteins form within the lumen of vacuoles through phase separation at specific stages of development. These droplets wet the tonoplast (vacuolar membrane), mediate tonoplast remodeling and formation of protein storage vacuoles (PNAS 2021, JCB 2021).
The group follows a collaborative approach and employs a broad spectrum of methods, ranging from cell biology and mathematical modelling to biophysics, interfacial sciences and synthetic biology. Drawing on the physiological relevance of in vivo data, our multi-faceted approach will reveal fundamental insights into the physicochemical mechanisms that link phase behaviour to morphogenic processes in cells.
(Keywords: membrane bending, membrane remodelling, membrane shaping, phase separation, LLPS, condenstates, wetting, capillarity, capillary force)
