RESEARCH HIGHLIGHTS
A new role for Ni in CO2 activation (Nature Catalysis, August 2025)
Yudhajeet Basak, Jae-Hun Jeoung, and Holger Dobbek, together with Christian Lorent and Ingo Zebger (TU Berlin)(UniSysCat), demonstrated that the nickel ion in the active site of carbon monoxide dehydrogenase (Ni,Fe-CODH) is the key player in CO2 activation and conversion. Using a combination of X-ray diffraction and spectroscopy on CODH crystals, they succeeded for the first time in visualizing all catalytically relevant states with bound reaction partners in the enzyme at atomic resolution.
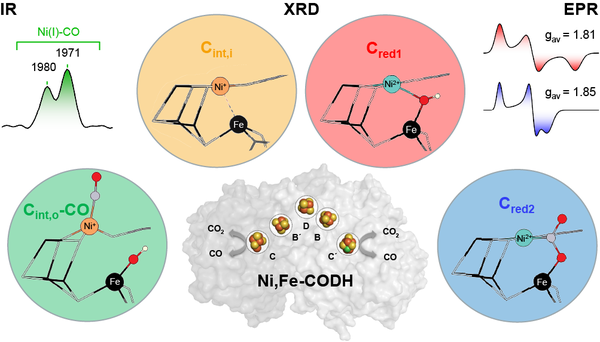
Yudhajeet Basak, Christian Lorent et al (2025) Nature Catalysis DOI:10.1038/s41929-025-01388-5
Hydrogen positions and water networks in photosystem II revealed by cryo-electron microscopy (Science, June 2024)
Rana Hussein together with Holger Dobbek, Athina Zouni and Julia Gaetcke (UniSysCat/SFB1078), and Petra Wendler (UniSysCat) in collaboration with colleagues from the Umeå and Uppsala Universities (Sweden) used cryo-electron microscopy single-particle analysis (cryo-EM SPA) to reveal previously undetected water networks and more than half of the hydrogen and proton positions. The results elucidate the water binding/activation and plastoquinone B protonation.
Rana Hussein, André Graça, et al (2024) Science DOI: 10.1126/science.adn6541
Evidence for the evolution of a tunnel to sequester CO, not to maximize its transport (Angewandte Chemie, May 2024)
Jakob Ruickoldt, Jae-Hun Jeoung, Julian Kreibich, Frank Lennartz, and Holger Dobbek together with colleagues from the group of Reinhard Schomäcker (UniSyscat, TU Berlin, Germany) used X-ray protein crystallography, phylogenesis, and kinetic methods to studied the properties of the CO transport tunnel connecting the two catalytic sites in the bifunctional complex CODH/ACS: reduction of CO2 in CODH and condensation of CO with a methyl moiety and CoA to acetyl-CoA in ACS. The results show that the complex CODH/ACS evolved the tunnel to sequester CO rather than to maximize turnover.
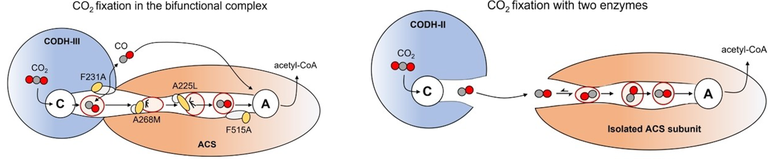
Jakob Ruickoldt, Jae-Hun Jeoung, Maik A. Rudolph, et al (2024) Ang. Int Ed Chem DOI: 10.1002/anie.202405120
Structural evidence for intermediates during O2 formation in photosystem II (Nature, May 2023)
Rana Hussein and Mohamed Ibrahim (present address Inst Mol Medicine, University of Lübeck, Germany) together with Holger Dobbek and Athina Zouni (UniSysCat/SFB1078) in collaboration with colleagues from the Lawrence Berkeley National Laboratory (CA, USA), Uppsala University (Sweden), KTH Royal Inst of Technology Stockholm, Sweden), RIKEN Spring-8 Center (Hyogo, Japan), SLAC National Accelerator Laboratory (Menlo Park, CA, USA), UCSF (San Francisco, CA, USA), UC Berkeley (CA, USA), UW Madison (Wisconsin, USA), Umeå University (Umeå, Sweden) used time-resolved crystallography (XFEL) to reveal groundbreaking structural insights into the mechanism of the water oxidation process of PSII during the S3-S0 transition.
Asmit Bhowmick, Rana Hussein, Isabel Bogacz, et al (2023) Nature DOI: 10.1038/s41586-023-06038-z
Discovery of the Lanthipeptide Curvocidin and Structural Insights into its Trifunctional Synthetase CuvL (Angew. Chem. Int. Ed, April 2023)
Berta Martins, María González-Viegas, and Holger Dobbek joined forces with colleagues from Roderich Süssmuth's group (UniSysCat, TU Berlin, Germany) to elucidate the principles of domain organisation and substrate recruitment of class IV and III lanthipeptide synthases. They used X-ray crystallography and NMR together with other biochemical tools.
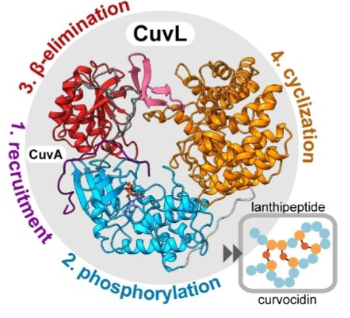
Arnar Sigurdsson, Berta M Martins et al., Ang. Int Ed Chem, e202302490, DOI: 10.1002/anie.202302490
Substrate Activation at the Ni,Fe Cluster of CO Dehydrogenases: The Influence of the Protein Matrix (ACS Catalysis, October 2022)
Yudhajeet Basak and colleagues analysed several variants of Ni,Fe-CODH. They showed that residues in the second coordination sphere of cluster C determine substrate activation and its coordination and stability. The study revealed how precariously the water structure at cluster C depends on the surrounding amino acids and how even seemingly minor changes can destabilise the cluster to the extent that asymmetrically coordinated [Fe4(μ3-S)4] clusters can form, highlighting the plasticity of cluster C.
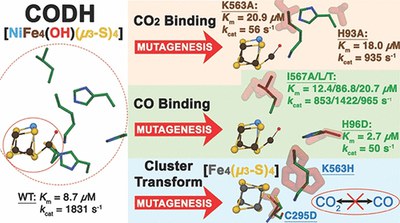
Yudhajeet Basak, Jae-Hun Jeoung, Lilith Domnik, Jakob Ruickoldt, and Holger Dobbek (2022) ACS Catalysis. 12711. DOI: 10.1021/acscatal.2c02922
Structural basis for coupled ATP-driven electron transfer in the double-cubane cluster protein (PNAS, June 2022)
Jae-Hun Jeoung, Sabine Nicklisch, and Holger Dobbek conducted binding and kinetics studies with X-ray crystallography to investigate the spatial arrangements of functional elements necessary for ATP-dependent electron transfer. Striking similarities with the non-homologous nitrogenases suggest a convergent evolution of catalytic strategies to achieve ATP-driven electron transfers between iron-sulfur clusters.
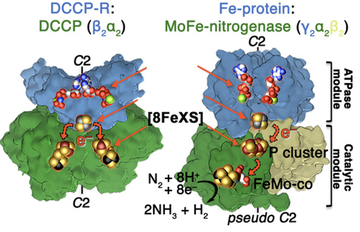
Jae-Hun Jeoung, Sabine Nicklisch and Holger Dobbek (2022) PNAS, 119, e2203576119, DOI:10.1073/pnas.2203576119
How a protein performs uphill electron transfer for reducing inert metabolites (ACS Catalysis, June 2021)
Felix Neumann and Holger Dobbek used enzymatic kinetics to explore how the ATP-dependent electron transfer between the metal-APTase reductive activator of CoFeSP (RACo) and its B12-dependent partner (CoFeSP) takes place. The results provide a blueprint for the efficient use of the energy of ATP in a coupling scheme involving conformational changes to generate a unidirectional uphill electron transfer.
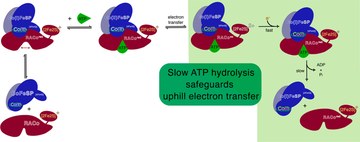
Felix Neumann and Holger Dobbek (2021) ACS Catalysis, 11, 8565 DOI:10.1021/acscatal.1c01038
Untangling the sequence of events during the S2 -> S3 transition in photosystem II and implications for the water oxidation mechanism (PNAS, May 2020)
Mohamed Ibrahim and Rana Hussein together with Holger Dobbek and Athina Zouni (UniSysCat/SFB1078) in collaboration with colleagues from University of Heidelberg (Germany), the Lawrence Berkeley National Laboratory (CA, USA), Uppsala University (Sweden), SLAC National Accelerator Laboratory (Menlo Park, CA, USA), Umeå University (Umeå, Sweden), Diamond Light Source Ltd (UK), Rutherford Appleton Laboratory (UK), RIKEN Spring-8 Center (Hyogo, Japan), UCSF (San Francisco, CA, USA), and UC Berkeley (CA, USA) used time-resolved crystallography (XFEL) to untangle the sequence of events during the S2 -> S3 transition.
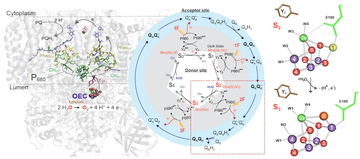
Mohamed Ibrahim, M, Thomas Fransson, Ruchira Chatteerjee, Mun Hon Cheah et al (2020) PNAS DOI:10.1073/pnas.2000529117
Where does the H2 go? X-ray crystallography and Vibrational Spectroscopy Reveal the Key Determinants of Biocatalytic Dihydrogen Cycling by [NiFe] Hydrogenases (Angew. Chem. Int. Ed, October 2019)
Yulia Ilina, Jae-Hun Jeoung and Holger Dobbek together with colleagues from the group of Ingo Zegber (Unicat, TU Berlin, Germany) used X-ray crystallography and vibrational spectroscopy to show that the protein matrix tunes the [NiFe] active site for efficient H2 binding and conversion.
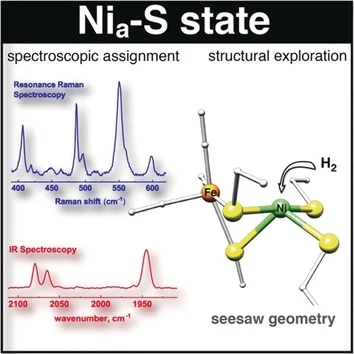
Yulia Ilina, Christian Lorent, Sagde Katz, Jae-Hun Jeoung, Seigo Shima, Marius Horch, Ingo Zebger and Holger Dobbek (2019) Ang. Int Ed Chem, 58, 18299 DOI: 10.1002/anie.201908258
A novel [Fe8S9] double-cubane cluster for ATP-dependent substrate reduction (PNAS, March 2018)
Jae-Hun Jeoung and Holger Dobbek used bioinformatic tools, kinetics and X-ray crystallography to describe a novel two-component enzyme catalyzing the chemically demanding reduction of small molecules (acetylene, azide, hydrazine), reactions so far known to be the hallmark of nitrogenases.
The two components are the DCCP (double-cubane cluster protein) and its reductase, DCCP-R (R for reductase). DCCP-R energises electrons at the expense of ATP hydrolysis. These electrons are then transferred to the active site [Fe8S9] double-cubane cluster in DCCP to enable the reduction of small molecule substrates. Although known from synthetic inorganic chemistry, this double-cubane cluster is unprecedented in biology. Comparison with members of the same ATP-dependent energizing electron systems suggests DCCP/DCCP-R is the first member of a novel enzyme family.
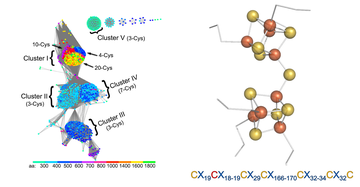
Jae-Hun Jeoung and Holger Dobbek (2018) PNAS, 115, 2994 DOI: 10.1073/pnas.1720489115
A catalytic active Michaelis-complex shows its secrets (Nature Communications, July 2017)
Tobias Werther and Holger Dobbek together with colleagues from the group of Peter Hildebrandt (Unicat, TU Berlin, Germany) used X-ray crystallography and resonance Raman spectroscopy to investigate the Michaelis complex of xenobiotic reductase A (reactive reduced cofactor bound to its substrates) and compare it to the non-reactive oxidized Michaelis complex mimics. They found that substrates bind in different orientations to the oxidized and reduced flavin, in both cases flattening its structure. But only authentic Michaelis complexes display an unexpectedly rich vibrational band pattern uncovering a strong donor-acceptor complex between reduced flavin and substrate. This interaction likely activates the catalytic ground state of the reduced flavin, accelerating the reaction within a compressed cofactor–substrate complex.
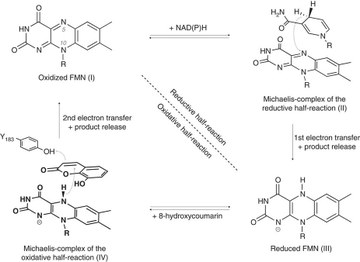
Tobias Werther, Stefan Wahlefeld, Johannes Salewski, Uwe Kuhlmann, Ingo Zebger, Peter Hilderbrandt and Holger Dobbek (2017) Nature Communications, 8, 16084. DOI: 10.1038/ncomms16084
Activation of dioxygen at a side-on O2-Nickel complex (Angew. Chem. Int. Ed, February 2016)
Jae-Hun Jeoung and Holger Dobbek together with colleagues from the group of Susanne Fetzner (Westfälische Wilhelms-Universität Münster, Germany) describe the catalytic role of nickel in quercetinase (QueD). QueD belongs to the cupin superfamily, which uses a conserved 3-His-1-Glu motif to bind a metal ion. Previous work from S. Fetzner's group pointed out that QueD shows the highest activity with nickel(2+), an unusual cofactor for oxygenases. They used a crystalographic cryotrapping approach to show how the binding of quercetin primes Ni(2+) to bind in side-on mode dioxygen, ready to react with quercetin.
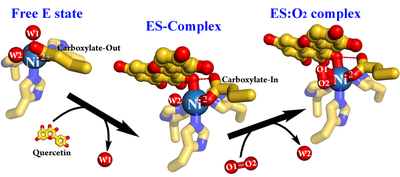
Jae-Hun Jeoung. Dimitrios Nianios, Susanne Fetzner, and Holger Dobbek (2016) Angew. Chem. Int. Ed., 55, 3281. DOI: 10.1002/anie.201510741
A blueprint for the activation of Carbon Dioxide (Angew. Chem. Int. Ed, July 2015)
Jochen Fesseler, Jae-Hun Jeoung, and Holger Dobbek describe in unprecedented detail how the activation of CO2 and its isoelectronic analog NCO– is achieved at a complex biological metal center. Carbon monoxide dehydrogenase (CODH) employs a [NiFe4S4] cluster (cluster C) to bind and activate CO2, which is subsequently split into CO and water. Using x-ray crystallography, the authors describe the geometry of the clusters with bound substrate CO2 and its inhibitor NCO– under turnover conditions at true-atomic resolution (dmin < 1.1 Å).
„Our insights will be of great help for synthetic inorganic and theoretical chemists“, explains Jochen Fesseler, lead author of the study. “By using our high-resolution structures as a blueprint, complexes with comparable catalytic power could be developed for large-scale CO2 conversions in the future.“
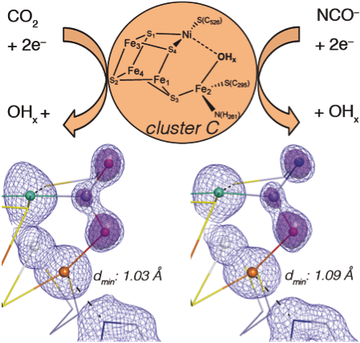
Jochen Fesseler, Jae-Hun Jeoung, and Holger Dobbek (2015) Angew. Chem. Int. Ed., 54, 8560 DOI: 10.1002/anie.201501778 (English) ; DOI: 10.1002/ange.201501778 (german)
How bacteria respires organohalide pollutants (Science, October 2014)
Martin Bommer, Jochen Fesseler, and Holger Dobbek together with colleagues from the group of Gabriele Diekert (Jena University, Germany) describe for the first time the crystal structure of PceA, an archetypal dehalogenase from Sulfurospirillum multivorans, a organohalide-respiring microorganism.
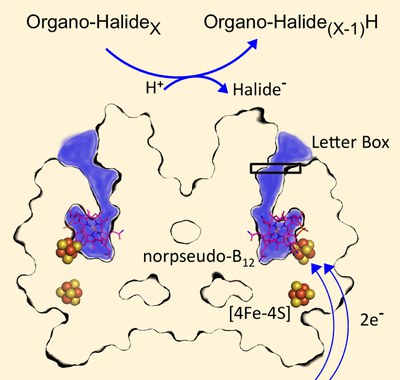
Martin Bommer, Cindy Kunze, Jochen Fesseler, Thorsten Schubert, Gabriele Diekert, and Holger Dobbek (2014) Science, 346, 455 DOI:10.1126/science.1258118
