Bacterial C1 metabolism
Reductive Acetyl-CoA pathway
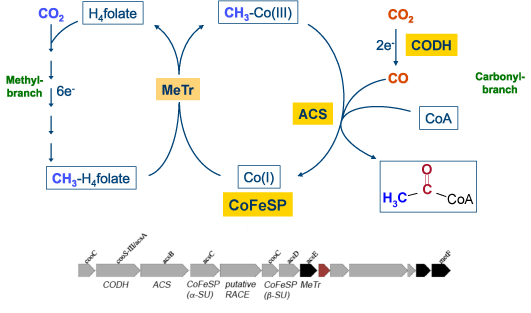
The carbon oxides CO and CO2 are used as a carbon source by several different types of microorganisms. Microbes living under anaerobic conditions can reduce CO2 directly to CO, which is used within the reductive acetyl-CoA pathway to synthesize acetyl-CoA.
We investigate the mechanism of the enzymes of this pathway and study the role of their accessory proteins:
Carbon monoxide dehydrogenase - CODH
Ni,Fe-containing carbon monoxide dehydrogenases catalyze the reversible reduction of CO2 to CO. The active site of carbon monoxide dehydrogenases contains a [NiFe4S4OH] cluster, which binds and converts CO and CO2. We want to determine the mechanism of this enzyme and want to find out why the enzyme is able to reduce CO2 without an over potential.
CooC-ATPases
The [NiFe4S4OH] cluster of CODHases is assembling spontaneously, but requires helper proteins. To insert Ni2+ into the heterometal cluster the ATPase CooC is needed, whose structure and function we are investigating.
Acetyl-coenzyme A synthase - ACS
Acetyl-Coenzyme A synthases catalyze the condensation of CO, coenzym A and a methyl cation. This reaction is catalyzed at a Ni-Ni-[4Fe4S] cluster- Details of the reaction mechanism are hardly understood.
Corrinoid iron/sulfur protein - CoFeSP
The corrinoid iron/sulfur protein serves as a methyl-transfer agent between the methyltetrahydrofolate dependent methyltransferase and acetyl-CoA synthase. CoFeSP allows a unique methyltransfer between two metal centres.
