Light-harvesting-like 3 (LIL3) proteins in Arabidopsis
Maxi Rothbart, Peng Wang, Daniel Hey, Jakob Müller
LIL3 double mutants (lil3.1/lil3.2) were found to accumulate only low amounts of geranylgeranylated chlorophyll instead of phytylated chlorophyll. Lack of LIL3 destabilized the geranylgeranyl reductase (GGR) in plastid membranes, which is encoded in the CHLP gene (Tanaka et al., 2010). It was concluded that physical interaction of LIL3 with CHLP ties the enzyme to the plastid membranes. CHLP catalyzes the reduction of geranylgeranyl either esterified with phosphate or with chlorophyllide and provides the hydrophobic carbohydrate chain for chlorophyll and tocopherols.
In continuation to previous work, we aim at to examine Lil3 function in chlorophyll biosynthesis. We hypothesize that LIL3 is important by intervening in the protein complex formation among the enzymes of late steps of chlorophyll biosynthesis and, whereby, substrate channeling is promoted.
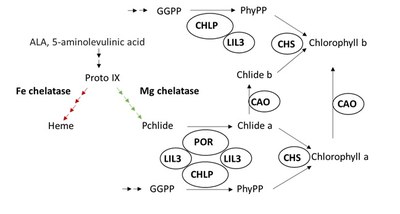
Model of the involvement of LIL3 in chlorophyll biosynthesis. The protochlorophyllide (Pchlide) conversion into chlorophyllide (Chlide) is catalyzed by protochlorophyllide oxidoreductase (POR). The geranylgeranyl reductase reduces geranylgeranyl-pyrophosphate (GGPP) to phytyl pyrophosphate. (PhyPP). These two enzymatic reductions are proposed to be united by means of the LIL3 protein for the subsequent reaction of the chlorophyll synthase (CHS). CAO, chlorophyll a oxygenase.
