Channelrhodopsins: light-activated ion channels
Channelrhodopsins (ChRs) are light-gated cation channels and function as primary photoreceptors in motile green algae. In the algae, ChRs are expressed in a specialized compartment - the so-called eyespot- where light initiates a fast inward-directed photocurrent. The electrical signal is amplified by the activation of voltage-gated secondary channels and is transmitted to the two flagella which in turn adjust their beating plane, frequency and pattern. Hence, the complex interplay of photoreceptors and flagella movement enables the algae to perform positive or negative phototaxis according to the quality of the ambient light.
The first two channelrhodopsins to be identified were the two isoforms from Chlamydomonas reinhardti, namely ChR1 and ChR2 [1-4]. Subsequently, further ChRs were found in related algae including Volvox cateri (VChR1 and VChR2), Dunaliella salina (DChR1) and Mesostigma viride (MChR1) [5-9]. All ChRs share a common architecture comprising an N-terminal membrane-spanning domain and a C-terminal cytosolic domain. Heterologous expression in conjunction with electrophysiological measurements showed that the membrane-spanning domain itself is sufficient to drive photocurrents. Thus, the putative channel is formed by the seven transmembrane helices, whereas the retinal chromophore is bound via a conserved lysine residue in helix seven (Figure 2a).
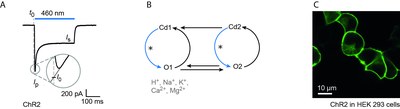
Fig. 1:
Upon activation with blue or green light channelrhodopsins undergo a typical photocycle that can be analyzed by time-resolved absorption and vibrational spectroscopy [10, 11]. Photon absorption triggers isomerization of the retinal from the all-trans configuration to the 13-cis form (Figure 2b). Subsequently, major structural changes of the protein backbone occur and a "preopen" state is formed on a sub-nanosecond timescale. Similar to proton-pumping rhodopsins, the retinal Schiff-base transiently deprotonates, thereby forming a blue-shifted photocycle intermediate (P390). Minor rearrangements of amino acids in the channel region accompanied by Schiff-base reprotonation leads to channel opening (P520). Finally, ChRs revert to the closed, dark state (D470) via several non-conducting late photocycle intermediates.
ChRs conduct cations with a relatively high selectivity for protons. Under physiological conditions ChRs may also conduct Na+, K+, Mg2+ and Ca2+ [3, 12, 13]. A typical photocurrent trace of ChR2 at negative voltages and high light intensities is depicted in Figure 1a. Photocurrents are characterized by an early peak current Ip that relaxes to a stationary current level Is during continuous illumination. In order to explain ChR inactivation, transient peak recovery and the different ion selectivities of early and late photocurrents a model with two open and two closed states has been proposed (Figure 1b) [13, 14, 15 ].
During the last decade large mutational screens have revealed a number of ChR mutants with modified properties. By replacing the corresponding residue to the primary proton acceptor in bacteriorhodopsin, fast-cycling ChR2 mutants (Cheta) have been created [16]. These variants are not only characterized by higher opening and closing rates, but also display voltage-insensitivity of the kinetics and a red-shifted activation maximum [17, 18]. Complementary to the Cheta mutants, slow-cycling ChRs were obtained by mutation of ChR2 C128 and D156 [19, 20]. These so-called step-function rhodopsins (SFRs) exhibit up to 10,000-fold decelerated photocycle kinetics. Moreover,ChR2-based SFRS can be turned on and off using alternating blue and yellow light pulses. Exchange of the nearby residue T159 to a cysteine leads to a ChR-variant with enhanced membrane expression and increased retinal binding affinity [17, 21]. Similarly, the ChR2 H134R mutant shows enhanced stationary photocurrents in different expression systems [22]. Other mutations in the retinal binding pocket and the channel region were shown to alter either the action spectrum, the kinetics or the ion selectivity of the respective mutant. Only recently, a chloride-selective ChR variant could be obtained by combination of the ChR2 E90R mutation with the two gain-of-function mutations T159C and D156N [23].
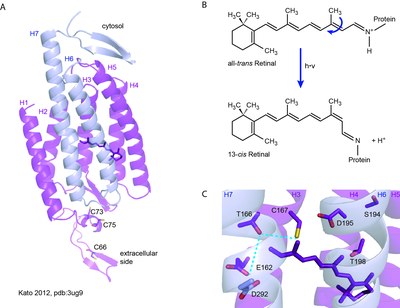
Fig. 2:
New ChR variants were also obtained by the chimera approach that combines helices from different ChRs [24,25,26]. The C1C2 chimera represents the first ChR that could be crystallized to obtain a high-resolution structure [27] (Figure 2a, 2c). Other chimeric ChRs such as C1V1 and ReachR combine red-shifted action spectra with enhanced membrane targeting and expression level [28, 29]. In the emerging field of optogenetics, ChRs are genetically targeted to the cell type of interest. Consequently, plasma membranes of target cells can be depolarized by light application. In neurons light-induced depolarization can be used to elicit action potentials with high spatiotemporal resolution [30]. Neuronal ChR activation has been used to study synaptic plasticity, to map neuronal connectivity and to alter behavior in different model organisms [22, 31-35]. Future clinical application of ChRs may include vision restoration, the repair of hearing impairments and the treatment of Parkinson's disease [36, 37, 38].
References
(1) O. A. Sineshchekov, K.-H. Jung, and J. L. Spudich,"Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii.", Proceedings of the National Academy of Sciences of the United States of America, vol. 99, pp. 8689-94, June 2002.
(2) G. Nagel, D. Ollig, M. Fuhrmann, S. Kateriya, A. M. Musti, E. Bamberg, and P. Hegemann, "Channelrhodopsin-1: a light-gated proton channel in green algae.," Science, vol. 296, pp. 2395-8, June 2002.
(3) G. Nagel, T. Szellas, W. Huhn, S. Kateriya, N. Adeishvili, P. Berthold, D. Ollig, P. Hegemann, and E. Bamberg, "Channelrhodopsin-2, a directly light-gated cation-selective membrane channel.," Proceedings of the National Academy of Sciences of the United States of America, vol. 100, pp. 13940-5, Nov. 2003.
(4) T. Suzuki, K. Yamasaki, S. Fujita, K. Oda, M. Iseki, K. Yoshida, M.Watanabe, H. Daiyasu, H. Toh, E. Asamizu, S. Tabata, K. Miura, H. Fukuzawa, S. Nakamura, and T. Takahashi, "Archaeal-type rhodopsins in Chlamydomonas: model structure and intracellular localization," Biochemical and Biophysical Research Communications, vol. 301, pp. 711-717, Feb. 2003.
(5) O. P. Ernst, P. A. S_anchez Murcia, P. Daldrop, S. P. Tsunoda, S. Kateriya, and P. Hegemann,"Photoactivation of channelrhodopsin.,"The Journal of biological chemistry, vol. 283, pp. 1637-43, Jan. 2008.
(6) F. Zhang, M. Prigge, F. Beyriere, S. P. Tsunoda, J. Mattis, O. Yizhar, P. Hegemann, and K. Deisseroth, "Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri.," Nature neuroscience, vol. 11, pp. 631-3, June 2008.
(7) A. Kianianmomeni, K. Stehfest, G. Nematollahi, P. Hegemann, and A. Hallmann, "Channelrhodopsins of Volvox carteri are photochromic proteins that are specifically expressed in somatic cells under control of light, temperature, and the sex inducer.," Plant physiology, vol. 151, pp. 347-66, Sept. 2009.
(8) F. Zhang, J. Vierock, O. Yizhar, L. E. Fenno, S. Tsunoda, A. Kianianmomeni, M. Prigge, A. Berndt, J. Cushman, J. Polle, J. Magnuson, P. Hegemann, and K. Deisseroth, "The microbial opsin family of optogenetic tools.," Cell, vol. 147, pp. 1446-57, Dec. 2011.
(9) E. G. Govorunova, E. N. Spudich, C. E. Lane, O. A. Sineshchekov, and J. L. Spudich, "New channelrhodopsin with a red-shifted spectrum and rapid kinetics from Mesostigma viride.," mBio, vol. 2, pp. e00115-11, Jan. 2011.
(10) E. Ritter, K. Stehfest, A. Berndt, P. Hegemann, and F. J. Bartl, "Monitoring light-induced structural changes of Channelrhodopsin-2 by UV-visible and Fourier transform infrared spectroscopy.," The Journal of biological chemistry, vol. 283, pp. 35033-41, Dec. 2008.
(11) K. Stehfest and P. Hegemann, "Evolution of the channelrhodopsin photocycle model.," Chemphyschem : a European journal of chemical physics and physical chemistry, vol. 11, pp. 1120-6, Apr. 2010.
(12) A. Berndt, M. Prigge, D. Gradmann, and P. Hegemann, "Two open states with progressive proton selectivities in the branched channelrhodopsin-2 photocycle.," Biophysical journal, vol. 98, pp. 753-61, Mar. 2010.
(13) F. Schneider, D. Gradmann, and P. Hegemann, "Ion Selectivity and Competition in Channelrhodopsins," Biophysical Journal, vol. 105, pp. 91-100, July 2013.
(14) P. Hegemann, S. Ehlenbeck, D. Gradmann, "Multiple photocycles of channelrhodopsin," Biophysical Journal, vol. 89, pp. 3911-8, Sep. 2005.
(15) K. Nikolic, N. Grossman, M. S. Grubb, J. Burrone, C. Toumazou, and P. Degenaar, "Photocycles of channelrhodopsin-2.," Photochemistry and photobiology, vol. 85, no. 1, pp. 400-11, 2009. (16) L. A. Gunaydin, O. Yizhar, A. Berndt, V. S. Sohal, K. Deisseroth, and P. Hegemann, "Ultrafast optogenetic control.," Nature neuroscience, vol. 13, pp. 387-92, Mar. 2010.
(17) A. Berndt, P. Schoenenberger, J. Mattis, K. M. Tye, K. Deisseroth, P. Hegemann, and T. G. Oertner, "High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels," Proc Natl Acad Sci U S A, vol. 108, no. 18, pp. 7595-7600, 2011.
(18) H. C. Watanabe, K. Welke, F. Schneider, S. Tsunoda, F. Zhang, K. Deisseroth, P. Hegemann, and M. Elstner, "Structural model of channelrhodopsin.," The Journal of Biological Chemistry, vol. 287, no. 10, pp. 7456-7466, 2012.
(19) A. Berndt, O. Yizhar, L. A. Gunaydin, P. Hegemann, and K. Deisseroth, "Bi-stable neural state switches.," Nature neuroscience, vol. 12, pp. 229-34, Feb. 2009.
(20) C. Bamann, R. Gueta, S. Kleinlogel, G. Nagel, and E. Bamberg, "Structural guidance of the photocycle of channelrhodopsin-2 by an interhelical hydrogen bond.," Biochemistry, vol. 49, pp. 267-78, Jan. 2010.
(21) S. Ullrich, R. Gueta, and G. Nagel, "Degradation of channelopsin-2 in the absence of retinal and degradation resistance in certain mutants.," Biological chemistry, Nov. 2012.
(22) G. Nagel, M. Brauner, J. F. Liewald, N. Adeishvili, E. Bamberg, and A. Gottschalk, "Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses.," Current biology, vol. 15, pp. 2279-84, Dec. 2005
(23) J. Wietek, J.S. Wiegert, N. Adeishvili, F. Schneider, H. Watanabe, S.P. Tsunoda, A. Vogt, M. Elstner, T.G. Oertner, P. Hegemann, "Conversion of channelrhodopsin into a light-gated chloride channel.," Science, vol. 344, no. 6182, pp. 409-12, Apr. 2014.
(24) H. Wang, Y. Sugiyama, T. Hikima, E. Sugano, H. Tomita, T. Takahashi, T. Ishizuka, and H. Yawo, "Molecular determinants differentiating photocurrent properties of two channelrhodopsins from chlamydomonas., "The Journal of biological chemistry, vol. 284, pp. 5685-96, Mar. 2009.
(25) S. P. Tsunoda and P. Hegemann, "Glu 87 of channelrhodopsin-1 causes pH-dependent color tuning and fast photocurrent inactivation.," Photochemistry and photobiology, vol. 85, no. 2, pp. 564-9, 2009.
(26) J. Y. Lin, M. Z. Lin, P. Steinbach, and R. Y. Tsien, "Characterization of engineered channelrhodopsin variants with improved properties and kinetics.," Biophysical journal, vol. 96, pp. 1803-14, Mar. 2009.
(27) H. E. Kato, F. Zhang, O. Yizhar, C. Ramakrishnan, T. Nishizawa, K. Hirata, J. Ito, Y. Aita, T. Tsukazaki, S. Hayashi, P. Hegemann, A. D. Maturana, R. Ishitani, K. Deisseroth, and O. Nureki, "Crystal structure of the channelrhodopsin light-gated cation channel.," Nature, vol. 482, pp. 369-74, Feb. 2012.
(28) O. Yizhar, L. E. Fenno, M. Prigge, F. Schneider, T. J. Davidson, D. J. O'Shea, V. S. Sohal, I. Goshen, J. Finkelstein, J. T. Paz, K. Stehfest, R. Fudim, C. Ramakrishnan, J. R. Huguenard, P. Hegemann, and K. Deisseroth, "Neocortical excitation/inhibition balance in information processing and social dysfunction.," Nature, vol. 477, no. 7363, pp. 171-178, 2011.
(29) J. Y. Lin, P. M. Knutsen, A. Muller, D. Kleinfeld, and R. Y. Tsien, "ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation.," Nature neuroscience, Sept. 2013.
(30) E. S. Boyden, F. Zhang, E. Bamberg, G. Nagel, and K. Deisseroth, Millisecond-timescale, genetically targeted optical control of neural activity.," Nature neuroscience, vol. 8, pp. 1263-8, Sept. 2005.
(31) F. Zhang, A. M. Aravanis, A. Adamantidis, L. de Lecea, and K. Deisseroth, "Circuitbreakers: optical technologies for probing neural signals and systems.," Nature reviews. Neuroscience, vol. 8, pp. 577-81, Aug. 2007.
(32) L. Petreanu, D. Huber, A. Sobczyk, and K. Svoboda, "Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections.," Nature neuroscience, vol. 10, pp. 663-8, May 2007.
(33) X. Li, D. V. Gutierrez, M. G. Hanson, J. Han, M. D. Mark, H. Chiel, P. Hegemann, L. T. Landmesser, and S. Herlitze, "Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin.," Proceedings of the National Academy of Sciences of the United States of America, vol. 102, pp. 17816-21, Dec. 2005.
(34) C. Schroll, T. Riemensperger, D. Bucher, J. Ehmer, T. Völler, K. Erbguth, B. Gerber, T. Hendel, G. Nagel, E. Buchner, and A. Fiala, "Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae.," Current biology , vol. 16, pp. 1741-7, Oct. 2006.
(35) B. R. Arenkiel, J. Peca, I. G. Davison, C. Feliciano, K. Deisseroth, G. J. Augustine, M. D. Ehlers, and G. Feng, "In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2.," Neuron, vol. 54, pp. 205-18, Apr. 2007.
(36) A. Bi, J. Cui, Y.-P. Ma, E. Olshevskaya, M. Pu, A. M. Dizhoor, and Z.-H. Pan,"Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration.," Neuron, vol. 50, pp. 23-33, May 2006.
(37) V. Gradinaru, M. Mogri, K. R. Thompson, J. M. Henderson, and K. Deisseroth, "Optical deconstruction of parkinsonian neural circuitry.," Science, vol. 324, pp. 354-9, Apr. 2009.
(38) T. Shimano, B. Fyk-Kolodziej, N. Mirza, M. Asako, K. Tomoda, S. Bledsoe, Z.-H. Pan, S. Molitor, and A. G. Holt, "Assessment of the AAV-mediated expression of channelrhodopsin-2 and halorhodopsin in brainstem neurons mediating auditory signaling.," Brain research, vol. 1511, pp. 138-52, May 2013.