Function of GUN4 in posttranslational regulation of the Mg chelatase reaction and ALA synthesis to sustain chlorophyll synthesis
Andreas Richter
Protein phosphorylation plays a crucial and diverse role in the control of activity and stability of proteins. Up to date only a few chloroplast localized protein kinases and their targets are identified. One of the most intriguing questions is still how protein phosphorylation contributes to the complex process of chloroplast biogenesis and regulation of biochemical pathways located in chloroplasts.
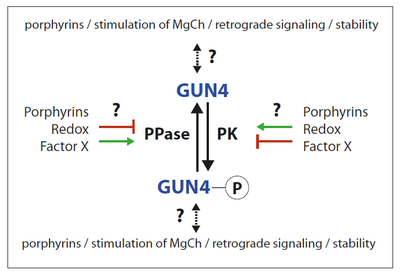
GUN4 is essential for chlorophyll synthesis and affects feedback control of ALA synthesis, the unique precursor for all tetrapyrroles. Knock out mutants of GUN4 cannot grow under photoperiodic growth, indicating the central role in the activation of the chlorophyll synthesis branch. GUN4 stimulates Mg chelatase (MgCh), the first committed enzymatic step in the chlorophyll synthesizing branch of tetrapyrrole biosynthesis in chloroplast. Although the function of this property is still not understood, GUN4 binds substrate and product of the MgCh reaction. Furthermore, GUN4 was shown to be involved in plastid-derived retrograde signaling, the communication pathway between the plastids and the nucleus.
The studies on the posttranslational regulation of GENOMES UNCOUPLED 4 (GUN4) aim to understand how the action of this post-translational regulator is influenced by phosphorylation (see figure). It is hypothesized that the involvement of GUN4 maintains adequate chlorophyll in adaptation to varying environmental conditions. Another objective is the identification of the GUN4-phosphorylating protein kinase.
The working program includes the following methods and experiments:
- Complementation assays of a gun4 knock out mutant expressing non-phosphorylatable and phosphomimicking GUN4
- in vitro analysis of recombinant MgCh activity in the presence of (non-)phosphorylated GUN4
- Porphyrin binding studies
- Protein-protein interaction studies
- Identification of the GUN4 kinase using biochemical separation and mass spectroscopy
- Computational modeling of 3D-protein structures