N-terminal protein modifications (NPM) - Methionine-aminopeptidase
Patrick Schall, Andreas Richter
Newly synthesized proteins are modified by a plethora of enzymes as soon as the first amino acids leave the ribosome exit tunnel. These N-terminal protein modifications (NPM) are ubiquitous in any living organism and are catalyzed by variety of dedicated enzymes (Figure 1). These enzymatic reactions alter the properties of the nascent proteins and transforms them into an active state. One of the most important enzymatic reactions in the NPMs is the cleavage of the first methionine by methionine aminopeptidases (MAPs), which increases the protein stability. In Arabidopsis thaliana, three MAPs isoforms – MAP1B, MAP1C and MAPC - have been identified, which are located to chloroplast and process over 40 % of all chloroplast encoded proteins.
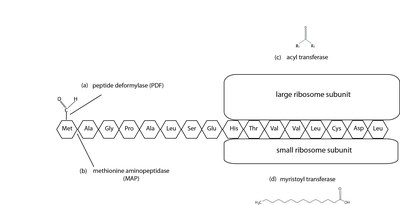
N-terminal protein modifications. (a) Peptide deformylases catalyze the cleavage of a formyl group from the initial methionine, which is necessary for further modification by MAPs (b). Methionine aminopeptidases remove the initial methionine from the nascent protein. (c) Acyl transferases add an acyl group to the nascent protein. (d) Myristoyl transferases add a myristoyl acid group to the growing protein.
Our recent focus is to study the specific roles of all three plastid-localized MAPs in A. thaliana. It seems all three isoforms recognize specific N-terminal target sites of their substrates. We aim to analyze in mutants and wild type seedlings the N-terminal modifications of specific target proteins for each of the three plastid-localized MAPs. We are interested to find out how the MAPs are posttranslationally regulated during the plant life cycle and under different environmental conditions and to which extent they have substrate specificity for plastid-encoded proteins.