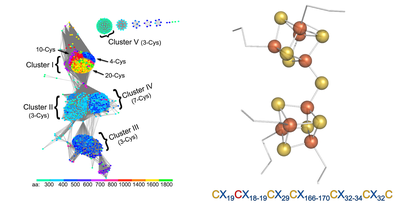
A novel [Fe8S9] double-cubane cluster for ATP-dependent substrate reduction
In their paper published in PNAS, Jae-Hun Jeoung and Holger Dobbek used bioinformatic tools, kinetics and x-ray crystallography to describe a novel two-component enzyme catalyzing the chemically demanding reduction of small molecules (acetylene, azide, hydrazine), reactions so far known to be the hallmark of nitrogenases.
The two components are the DCCP (double-cubane cluster protein) and its reductase, DCCP-R (R for Reductase). DCCP-R energizes electrons at the expense of ATP hydrolysis and these electrons are then used by the DCCP to reduce small molecule substrates at the active site [Fe8S9] double-cubane cluster. Although known from synthetic inorganic chemistry, this double-cubane cluster is unprecedented in biology. Comparison with members of the same ATP-dependent energizing electron systems, suggest it to be the first member of a novel enzyme family.
Citation:
Jae-Hun Jeoung and Holger Dobbek (2018): “ATP-dependent substrate reduction at an [Fe8S9] double-cubane cluster.“
PNAS doi: 10.1073/pnas.1720489115