Rhodopsins
Rhodopsins are one of the main photoreceptor types spread over all kingdoms of life. According to their amino acid sequence rhodopsins are classified into two groups. While type II rhodopsins function as G-protein coupled receptors mainly in metazoan vision, type I rhodopsin constitute light-guided ion pumps/channels or sensors for energy homeostasis, signal transduction and phototaxis in archaea, eubacteria and lower eukaryotes. Both rhodopsin types share the classical 7-transmembrane helical fold and harbor the organic retinal moiety covalently bound to helix 7, which serves as light-antenna (chromophore)1,2. Recently, a microbial rhodopsin class has been discovered with an exceptional multi-domain architecture and functionality. For these enzyme rhodopsins, the light sensing rhodopsin domain is directly connected to one or multiple output domains. The terminal effector domain can be enzymatic, ranging from adenylyl/guanylyl cyclases for cyclic nucleotide (cNMP) synthesis to phosphodiesterases for cNMP degradation. The cyclic nucleotides cAMP and cGMP are crucial 2nd messengers for a pleiotropy of cellular processes like gene regulation, cell growth, the visionary cascade, contractility, cardiovascular homeostasis, metabolism, cell growth, synaptic transmission and learning3,4.
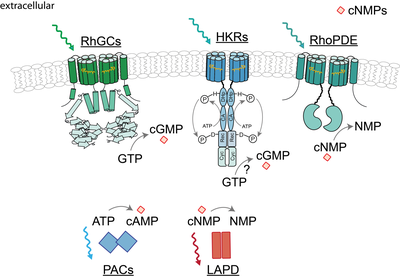
Fig.1 Overview of enzyme-coupled photoreceptors involved in cyclic nucleotide modulation.
These novel photoreceptors have been studied by us and others with regard to their light absorbing characteristics, their functionality and their structure. One representative of this new enzyme rhodopsin class has been discovered in aquatic fungi (Blastocladiamyota) and harbors a type III guanylyl cyclase domain connected via a 40 amino acid linker to a rhodopsin domain5. We and others found that green light activates the rhodopsin-guanylyl cyclases (RhGCs) to produce cGMP in various mammalian systems, while the enzyme isn’t active in darkness6–8. Thus, RhGCs are suitable optogenetic tools to modulate cGMP signaling. According to other type III cyclases, the homodimerization of two RhGC subunits is likely to assemble the catalytic unit. Similar to RhGC, a rhodopsin-phosphodiesterase (RhoPDE) has been discovered in the choanoflagellate Salpingoeca rosetta, which connects a phosphodiesterase to the rhodopsin domain via a linker of 50-60 amino acids. RhoPDE preferentially hydrolyses cGMP over cAMP but shows hydrolytic activity already in darkness. Illumination either resulted in minor or no increase of cNMP degradation9,10. Another focus of our research on microbial rhodopsins encompasses histidine kinase rhodopsins (HKRs), which harbor an even more complex architecture. In HKRs the rhodopsin domain is linked to a large cytosolic region with high homologies to the dimerization and phosphorylation domains (Dhp), the catalytical domains (CA) and the response regulator like receiver domains known from hybrid-histidine kinases. Therefore occurrence of a light-induced signal transduction cascade including autophosphorylation and phosphorelay is assumed. In some HKRs the receiver domain is additionally linked to a C-terminal guanylyl cyclase subunit, which is presumably regulated by the upstream elements. Spectroscopic studies of HKR-rhodopsin-domains from unicellular green algae revealed a pronounced photochromicity involving two long-living well-defined absorbance forms. Accordingly the environmental light situation controls the equilibrium between these isoforms 11,12. As one of these absorbance states is expected to induce the signal transduction, HKR-photoreceptor subunits are supposed to be highly efficient molecular switches, regulated by light.
Apart from membrane-targeted enzyme rhodopsins, soluble photoreceptors are widely applied to modulate the cellular cyclic nucleotide level with light, which share the modular domain architecture. In particular, the blue light activated adenylate cyclase from Beggiatoa (bPAC) represents today’s leading optogenetic tool to induce cAMP synthesis13,14 . In contrast to rhodopsins, the photoreception of bPAC is based on the blue light sensors using FAD (BLUF) domain, which precedes the adenylate cyclase. In complement to bPAC, the engineered light-activated phosphodiesterases (LAPD) allows cNMP hydrolysis, which can be activated with red light. Photoreception of LAPD is based on the phytochrome domain, preceding the phosphodiesterase13,15.
Involved in this project are:
Literature
1. Ernst, O. P. et al. Microbial and Animal Rhodopsins: Structures, Functions, and Molecular Mechanisms. Chem. Rev. 114, 126–163 (2014).
2. Spudich, J. L., Yang, C.-S., Jung, K.-H. & Spudich, E. N. Retinylidene Proteins: Structures and Functions from Archaea to Humans. Annu. Rev. Cell Dev. Biol. 16, 365–392 (2000).
3. Beavo, J. A. & Brunton, L. L. Cyclic nucleotide research — still expanding after half a century. Nat. Rev. Mol. Cell Biol. 3, 710–718 (2002).
4. Feil, R. & Kemp-Harper, B. cGMP signalling: from bench to bedside. Conference on cGMP generators, effectors and therapeutic implications. EMBO Rep. 7, 149–53 (2006).
5. Avelar, G. M. et al. A rhodopsin-guanylyl cyclase gene fusion functions in visual perception in a fungus. Curr. Biol. 24, 1234–40 (2014).
6. Scheib, U. et al. The rhodopsin-guanylyl cyclase of the aquatic fungus Blastocladiella emersonii enables fast optical control of cGMP signaling. Sci. Signal. 8, rs8 (2015).
7. Gao, S. et al. Optogenetic manipulation of cGMP in cells and animals by the tightly light-regulated guanylyl-cyclase opsin CyclOp. Nat. Commun. 6, 8046 (2015).
8. Trieu, M. M. et al. Expression, Purification, and Spectral Tuning of RhoGC, a Retinylidene/Guanylyl Cyclase Fusion Protein and Optogenetics Tool from the Aquatic Fungus Blastocladiella emersonii. J. Biol. Chem. jbc.M117.789636 (2017). doi:10.1074/jbc.M117.789636
9. Lamarche, L. B. et al. Purification and Characterization of RhoPDE, a Retinylidene/Phosphodiesterase Fusion Protein and Potential Optogenetic Tool from the Choanoflagellate Salpingoeca rosetta. Biochemistry 56, 5812–5822 (2017).
10. Yoshida, K., Tsunoda, S. P., Brown, L. S. & Kandori, H. A unique choanoflagellate enzyme rhodopsin exhibits light-dependent cyclic nucleotide phosphodiesterase activity. J. Biol. Chem. 292, 7531–7541 (2017).
11. Luck, M. et al. A photochromic histidine kinase rhodopsin (HKR1) that is bimodally switched by ultraviolet and blue light. J. Biol. Chem. 287, 40083–40090 (2012).
12. Luck, M. & Hegemann, P. The two parallel photocycles of the Chlamydomonas sensory photoreceptor histidine kinase rhodopsin 1. J. Plant Physiol. (2017). doi:10.1016/j.jplph.2017.07.008
13. Jansen, V., Jikeli, J. F. & Wachten, D. How to control cyclic nucleotide signaling by light. Curr. Opin. Biotechnol. 48, 15–20 (2017).
14. Stierl, M. et al. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J. Biol. Chem. 286, 1181–1188 (2011).
15. Gasser, C. et al. Engineering of a red-light-activated human cAMP/cGMP-specific phosphodiesterase. Proc. Natl. Acad. Sci. 111, 8803–8808 (2014).