Current state of research : Overview
As we all know, the global energy demand is steadily rising. Most of today's energy sources are either non-renewable, unsustainable or contribute to greenhouse gases in the environment. Solar energy is a clean alternative energy source, but the current methods of converting it into transportable fuels are costly and inefficient. However, in nature, solar energy is efficiently used through the process of photosynthesis in plants, cyanobacteria and algae. Our primary goal is to unravel the individual photosynthesis processes at the molecular level using various spectroscopic and X-ray structure analysis techniques. This fundamental research provides the basis for the application in artificial photosynthesis. Here, all research projects that are carried out in the AG Zouni are briefly summarized. We are working intensively with many national and international research groups on these projects in order to get an answer to the most urgent energy and environmental questions. A detailed description of the projects can be found under the respective: SFB 1078; UniSysCat C3; UniSysCat B2; BMBF.
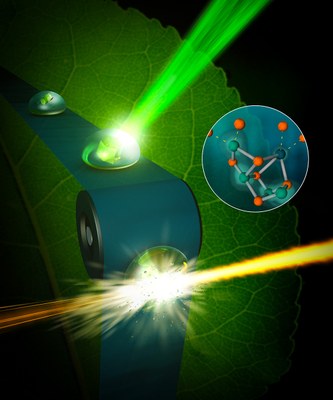
Figure 1: Femtosecond XFEL measurements at room temperature (LCLS-Stanford, USA) on our generated Photosystem II microcrystals at ca. 2.0 Å resolution. Hereby, the structures of the intermediates of Kok’s photosynthetic water oxidation clock could be elucidated (Kern et al., Nature, 2018). Picture source: https://www2.lbl.gov/vkyachan/index.html, date: April 12th, 2019

PHOTOSYSTEM II and atmospheric O2 EVOLUTION
The basic pillars of oxygenic photosynthesis in green plants, algae, and cyanobacteria are two membrane protein complexes, photosystem I (PSI) and photosystem II (PSII), which are built up of numerous subunits. The oxygen in the atmosphere is produced by the light-induced oxidation of water in PSII. The water oxidizing complex (WOC), which holds a Mn4CaO5-cluster, is catalyzing the reaction:
2 H2O → O2 + 4 e- + 4 H+
Our main objective is the determination of the mechanism of water splitting in PSII. This requires the identification of the substrate water molecules as well as the amino acid side chains and their protonation states in defined oxidation states of the WOC. The formation of atmospheric O2 in photosynthetic water oxidation at the Mn4CaO5-cluster in Photosystem-II is the key to solar fuel production. The SFB 1078 project (A5; Zouni/Dobbek) includes the following fields: See SFB 1078 for detailed information.

UniSysCat (Project C3.3)
An important aspect in PSII research is the repair cycle, in which key components of PSII, including the whole catalytic Mn4CaO5 cluster, are degraded and re-assembled approximately every half hour. This process is still completely unclear. Despite the recent progress in PSII structure elucidation (see Sfb1078 project), the mechanism of the light-triggered formation of the Mn4CaO5 cluster from its constituting ions remains elusive. Very recently, we have obtained a crystal structure of PSII that is completely depleted of the Mn4CaO5 cluster at a resolution of 2.5 Å (apo-dPSII) (M. Zhang et al, eLife, 2017). This structure can serve as a basis for understanding the mechanism of the WOC assembly. Within the UniSysCat cluster, a basic understanding of the dynamic water oxidation reaction in PSII under physiological conditions is essential for the design of artificial water oxidizing catalysts. Therefore, a systematic investigation of the photo-assembly of the Mn4CaO5 clusters based on apo-dPSII single crystals is planned to form and structurally characterize PSII intermediates during the assembly process. This research will also provide important information for the synthesis of artificial water splitting catalysts.
PHOTOSYSTEM I
UniSysCat project B2
It is of great environmental and economic interest to understand the principles of solar energy conversion in natural systems. A promising complex that is stable and converts solar energy with high quantum efficiency is Photosystem I (PSI). The aim of this project is the light-driven production of basic chemicals by coupling enzymes to PSI. Previous studies on the binding of different cytochrome variants under solution conditions as well as structural analyses of PSI-cyt co-crystals (Kölsch et al., JBC, 2018) will be continued by means of free-electron laser X-ray studies. The aim is to generate a light-driven enzymatic activity in solution that serves as a template for future applications.
BMBF Project 2020+
The project is a high-risk basic project, which will initially find fundamental solutions for the use of photobioelectrodes for the conversion of light energy into electrical and chemical energy in the form of recyclable materials. The main goal of the tandem project (AG Lisdat/TFH Wildau) is to design light-activated electrode assemblies for effective energy conversion. Within this project, work will be carried out to increase the efficiency of the energy conversion of photobioelectrodes as well as to develop new coupling strategies of PSI with enzymatic conversion reactions. The final goal is to achieve the use of solar energy for photocurrent generation in combination with the production of recyclable materials and to put it on a solid basis.
Our central research method is X-ray structure analysis at synchrotron radiation sources (BESSY, DESY, ESRF) and free-electron laser (XFEL) sources enabling time-resolved structure determination at room temperature (LCLS-Stanford, USA; SACLA, Japan; PAL; Korea). For proton determination in Photosystem II using Neutrondiffraction experiments (ONRL; Oak Ridge, USA) . In addition, we use X-ray absorption spectroscopy (XANES, EXAFS) and X-ray emission spectroscopy (XES). The structures and electronic properties of metal centers are determined with ps to ms time resolution. Density Functional Theory (DFT) calculations are used in a complementary way to obtain geometry-optimized model structures and spectral simulations, as well as deeper insights into electronic properties. Furthermore, we employ Membrane Inlet Mass Spetrometry (MIMS). Protein samples are prepared from the thermophilic photosynthetic organism Thermosynechococcus elongatus cultivated in a photobioreactor by using chromatographic methods (FPLC). Studies of the homogeneity of proteins by dynamic light scattering and other biochemical and biophysical methods to determine the degree of purity of proteins are in use, for example, Blue-Nativ-Gel, Westernblott, polarographic methods to determine the activity of proteins, etc. .
