Biocatalytic coupling of photosystem I with FDH and CODH supercomplexes
UniSysCat (2019 – 2022): EXC 314/1
Project B.2.2: A. Zouni
Principal Investigators: Prof. A. Zouni
Prof. A. Zouni: supervision of PhD (NN); TA G. Bartels
Abstract:
The sun is a clean energy source, and it is of interest to understand the principles of solar energy conversion in natural photosynthetic systems. A promising complex, being stable and converting solar energy with a high quantum efficiency, is photosystem I (PSI). The goal of the present project is the light-driven generation of basic chemicals through the coupling of enzymes like formate dehydrogenase (FDH) or carbon monoxide dehydrogenase (CODH) to PSI. To transfer electrons efficiently, we use a molecular wire that covalently binds the iron-sulfur cluster FB at the acceptor side of PSI to an iron-sulfur cluster of FDH or CODH. At its donor side, PSI takes up electrons from cytochrome c (cyt c). To better understand this electron transfer, previous investigations of the non-covalent binding of different cyt c variants under solution conditions as well as structural analyses of PSI-cyt c co-crystals (Kölsch et al., JBC, 2018) will be continued. Structures should be improved to a resolution of at least 2.5 Å for both synchrotron X-ray studies (BESSY, DESY, in collaboration with the Dobbek group) and free-electron laser X-ray studies (LCLS, Stanford, USA, with the Berkeley group).Very recently, we solved the cryo-EM structure of a cross-linked cyt c6 - PSI super complex (with the Wendler group) and investigate the solution structure by means of small-angle X-ray scattering (SAXS, in cooperation with J. Pieper) (A. Kölsch et al, 2020, submitted). From theoretical modeling, the docking sites for native cyt c6 became apparent. We want to improve this modeling as regards pH dependence and accuracy by taking into account protonation states of titratable groups in the proteins (in cooperation with F. Müh). The ultimate goal of the project is to generate light-triggered enzymatic activity of these super complexes in solution to serve as a template for artificial applications (Leimkühler, Dobbek).
Introduction:
Learning from nature, light-to-charge carrier converting proteins from oxygenic photosynthesis of plants and cyanobacteria are of high interest for the construction of new functional devices (Lubner C. E. et al, PNAS, 2011; Ciornii D. et al, Electrochim. Acta, 2019). One of the most promising light-converting complexes is photosystem I (PSI), because of its high quantum efficiency (~100 %), fast and stable charge separation, and a proper spectral overlap with our sun. PSI from the thermophilic cyanobacterium Thermosynechococcus elongatus (T. elongatus) is a trimeric pigment-protein super-complex consisting of 12 different protein subunits, harboring 96 chlorophylls a (Chl a) and 22 carotenoids per monomer. Most Chls serve as light-harvesting antenna pigments and 6 Chls form an electron transport chain (Jordan P. et al, Nature, 2001). PSI catalyzes the light-driven transfer of an electron from reduced cytochrome c (Cyt cred) at the luminal side to oxidized ferredoxin (Fdox) at the stromal side. The charge separation in PSI occurs between the Chl a/Chl a’ heterodimer, named P700, and the primary acceptor named A0. The electron is subsequently transferred to a bound phyllochinone and then serially through three [4Fe-4S] clusters, Fx, FA and FB, to a soluble [2Fe-2S] Fd. As part of the UniSysCat cluster, the main objective of this project, in close collaboration with the working groups Leimkühler, Dobbek, Wendler, is the production of basic chemicals with light as an energy source.To achieve these long-term goals, an artificial photosynthesis system will be established through coupling photochemical, PSI, and catalytic modules, e.g., formate dehydrogenase (FDH) or carbon monoxide dehydrogenase (CODH), for the construction of a light-driven, formate or CO evolving device. To transfer electrons efficiently and with a high quantum yield, we will use a molecular wire that covalently attaches the FB cluster of PSI to an iron-sulfur cluster of FDH or CODH (Fig. 1).
Einleitung (deutsch): Lernen von der Natur
Proteine der oxygenen Photosynthese von Pflanzen und Cyanobakterien sind als Licht-zu-Ladung konvertierende Carrier von großem Interesse für die Konstruktion neuer funktioneller (Bau-)Elemente (Lubner C. E. et al, PNAS, 2011; Ciornii D. et al, Electrochim. Acta, 2019). Wegen seiner hohen Quantenausbeute (100%), einer schnellen und stabilen Ladungstrennung und einer geeigneten Überlappung mit dem Sonnenspektrum ist Photosystem I (PSI) einer der vielversprechendsten Licht umwandelnden Komplexe. PSI des thermophilen Cyanobakteriums Thermosynechococcus elongatus (T. elongatus) ist ein trimerer Pigment-Protein-Superkomplex, welcher aus 12 verschiedenen Untereinheiten besteht und je Monomer 96 Chlorophyll a – Moleküle (Chl a) und 22 Carotinoide enthält. Die meisten Chlorophylle dienen als lichtsammelnde Antennenpigmente, wobei sechs der Chlorophylle die Elektronentransportkette bilden (Jordan P. et al, Nature, 2001). PSI katalysiert den lichtgetriebenen Elektronentransfer vom reduzierten Cytochrom c (Cyt cred) an der luminalen Seite zum oxidierten Ferredoxin (Fdox) auf der Stromaseite. Die Ladungstrennung im PSI erfolgt zwischen dem Chl a / Chl a´ Heterodimer, dem P700, und dem Primärakzeptor A0. Anschließend wird das Elektron zunächst auf ein gebundenes Phyllochinon, dann fortlaufend über drei [4Fe-4S] Cluster auf ein lösliches [2Fe-2S] Fd übertragen. Als Teil des UniSysCat-Clusters ist das Hauptanliegen dieses Projektes, in enger Zusammenarbeit mit den Arbeitsgruppen Leimkühler, Dobbek und Wendler, die Synthese von einfachen Chemikalien durch Lichtenergie. Um diese langfristigen Ziele zu erreichen, wird ein künstliches Photosynthese-System entwickelt. Diese Systeme bestehen aus dem photochemischen Modul, dem PSI, und einem katalytischen Modul, z.B. der Formiatdehydrogenase (FDH) oder der Kohlenmonoxid-Dehydrogenase (CODH), um ein lichtgetriebenes Formiat- oder Kohlenmonoxid-produzierendes Element aufzubauen. Um Elektronen effizient und mit einer hohen Quantenausbeute weiterzuleiten, soll eine kovalente Bindung des FB-Clusters von PSI an ein Eisen-Schwefel-Cluster von FDH oder CODH durch ein „molekulares Kabel“ erreicht werden (Abb. 1).
Recent results:
For the optimization of the light to formate or CO conversion, we will analyze and optimize the electron transfer of PSI on its both luminal and stromal sides.
The modification at the luminal site focuses on the optimized covalent coupling of the native electron donor cytochrome (cyt) c6 isolated from T. elongatus or the non-native cyt c from horse heart (cyt cHH) at the binding site of PSI from T. elongatus. However, we lack knowledge about the identity of the PSI-cyt c binding site which is necessary for successful modifications. For this approach, a series of binding studies of PSI with cyt c6 and cyt cHH including oxygen consumption measurements, isothermal titration calorimetry (ITC), and rigid body docking combined with electrostatic computations of binding energies has been performed optimizing the electron transfer from cyt c to PSI in solution. While PSI has a higher affinity for the cyt cHH than for cyt c6, the influence of the ionic strength and pH on binding is different in the two cases. ITC and theoretical computations reveal the existence of unspecific binding sites for cyt cHH, besides one specific binding site close to P700. Binding to PSI is found to be the same for reducing and oxidized cyt cHH. Based on this information, we were able to produce co-crystals consisting of cyt cHH with PSI in a ratio of 1:1. A crystal structure at 3.4 Å resolution has been determined, but cyt cHH cannot be identified in the electron density map because of unspecific binding sites and/or a high flexibility at the binding site (Kölsch et al., JBC, 2018). Modeling the binding of cyt c6 to PSI reveals a specific binding site where the distance and orientation of cyt c6 relative to P700 is comparable to cyt c2 from purple bacteria relative to P870.
Future plans and cooperation
At the stromal side, a hybrid complex, consisting of carbon monoxide dehydrogenase (CODH) or formate dehydrogenase (FDH) and PSI, will be constructed by fusion of CODH or FDH with the small, stromal subunit PsaC (Fig. 1).
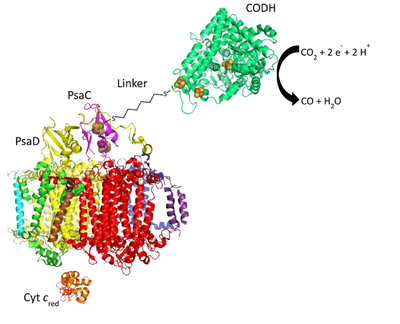
Fig.1: Assembly of the PSI-molecular wire-1,6-hexanedithiol-CODH bio-conjugate beginning with the Fe-S reconstituted Cys13Gly variant of unbound PsaC. Cyt c, PsaC, PsaD, molecular wire, and CODH are not scaled to the same dimension as the protein.
To achieve this goal, we are going to generate a PSI free of the three stromaly associated proteins - named PsaC, PsaD, and PsaE. Initial experiments to separate the three extrinsic proteins PsaC, D and E from the PSI complex have already been successfully performed by the addition of chemical reagents. The PSI without the three extrinsic proteins was analyzed by biochemical and biophysical measurement method. The protein shows a higher oxygen activity, and that all other subunits are completely preserved. This gives us the opportunity to achieve a direct coupling with FDH or CODH and to analyze the functionality of this protein-protein complex in cooperation with the Leimkühler or Dobbek group.
A second way for separating the three protein subunits from PSI is by interruption of the coding region of the psaC gene. Preliminary work showed a partial integration of the resistance cassette in the psaC gene. The complete recombination of the wildtype psaC gene will be achieved by increasing the antibiotic concentration. The modified PSI without the subunits PsaC, PsaD, and PsaE will be employed for reconstitution experiments using heterologously expressed PsaD and PsaC (C13G), which lacks a coordination site for the iron-sulfur cluster FB. A linker will be introduced between the FB cluster of PSI and the distal Fe-S cluster of CODH or FDH. By applying linkers with different length, the iron-sulfur clusters will be brought sufficiently close, so that forward electron transfer outcompetes charge recombination.
Cooperation partner:
- Holger Dobbek/HU Berlin
- Silke Leimkühler/Universität Potsdam
- Petra Wendler/ Universität Potsdam
- Jörg Pieper/University Tartu (Estland)
- Frank Müh/Keppler University (Linz, Austria)
- Vittal Yachandra/Junko Yano (Lawrence Berkeley National Laboratory (USA)
References
Lubner, C.E., Applegate, A.M., Knörzer, P., Ganago, A., Bryant, D.A., Happe, T., and Golbeck, J.H. (2011). Solar hydrogen-producing bionanodevice outperforms natural photosynthesis. Proceedings of the National Academy of Sciences 108, 20988–20991.
Jordan, P., Fromme, P., Witt, H. T., Klukas, O., Saenger, W., and Krauss, N. (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 A˚ resolution. Nature 411, 909–917.
Kern, J., Loll, B., Lüneburg, C., DiFiore, D., Biesiadka, J., Irrgang, K.D., and Zouni, A. (2005) Purification, characterization and crystallisation of photosystem II from Thermosynechococcus elogatus cultivated in a new type of photobioreactor. Biochem. Biophys. Acta 1706, 147-157.
Kern, J.; Müh, F.; Zouni, A., Structural studies on tetrapyrrole containing proteins enabled by femtosecond X-ray pulses. Metabolism, Structure and Function of Plant Tetrapyrroles: Control Mechanisms of Chlorophyll Biosynthesis and Analysis of Chlorophyll-Binding Proteins 2019, 33. https://bit.ly/2PyaVSz
Ciornii D, Kölsch A, Zouni A, Lisdat F. Exploiting new ways for a more efficient orientation and wiring of PSI to electrodes: A fullerene C70 approach. Electrochimica Acta. Volume 299, 2019, Pages 531-539.
https://doi.org/10.1016/j.electacta.2019.01.032
Kölsch A, Hejazi M, Stieger KR, Feifel SC, Kern JF, Müh F, Lisdat F, Lokstein H, Zouni A. Insights into the binding behavior of native and non-native cytochromes to Photosystem I from Thermosynechococcus elongatus. J Biol Chem. 2018 Jun 8;293(23):9090-9100. doi: 10.1074/jbc.RA117.000953.
Kölsch A et al, Current limits of structural biology: the transient interaction between cytochrome c6 and photosystem I, 2020, submitted.